Q: What is the origin of the 340B program?
A: The 340B program was enacted in the Public Health Service (PHS) Act of 1992. It allows qualified covered entities and federal grantees to purchase covered outpatient drugs at the Medicaid price or bill Medicaid, but not both (known as the duplicate discount rule).
Q: How big is the 340B program today?
A: According to the Drug Channels Institute, discounted purchases under the 340B program continue to grow, up to $38 billion in 2020. That number is 27% higher than $29.9 billion in 2019 and quadruple the value of discounted purchases in 2014.
Q: What are the latest developments in the 340B program from a manufacturer’s perspective?
A: Recently, the eligibility of contract pharmacies by some pharmaceutical manufacturers have been challenged as a source of duplicate or cumulative discounts. In the past year, a handful of manufacturers stopped providing 340B discounts for some or all prescriptions dispensed by contract pharmacies, while others limited sales by requiring specific data submissions or selling drug products only after a covered entity has demonstrated 340B compliance.
There are multiple lawsuits related to these actions since the elimination or reduction of these programs and services has the potential to impact public health. By deciding not to offer discounts, these drugs will not be made available to an underserved population, which reduces provider revenues and increases profits to manufacturers. This especially impacts underserved and uninsured populations, who would be disproportionately impacted by these service cuts.
Moreover, many industry commentators and practitioners are questioning whether the massive proceeds earned by covered entities are inflating the costs of commercial patients, and even worse whether under-insured patients are receiving the benefits desired when the 340B program was enacted.
Clearly, 340B evokes strong responses for and against the program; however, any modernization reforms will require new regulations to be enacted.
Q: Who monitors the 340B program?
A: The Health Resources and Services Administration (HRSA) has a program integrity oversight role. They perform audits on a small percentage of covered entities and manufacturers annually and can levy penalties or terminate participants from the program due to violations like duplicate discounts, diversions, or GPO prohibition.
Q: What is diversion?
A: Diversion is the transfer of drugs at 340B prices to ineligible patients. A few examples of how diversion happens are:
- Drug dispensed at a contract pharmacy for a prescription written at an ineligible site
- 340B drugs dispensed to ineligible patients
- Drug dispensed at an entity not supported by medical records
- Drug dispensed at a contract pharmacy with no documented provider/patient relationship

IntegriChain’s analysis shows that diversion is by far the most prevalent adverse finding but most difficult for pharmaceutical manufacturers to address. While diversion is declining, duplicate discounts are trending up over time.

On average 60% have historically resulted in sanctions such as manufacturer payment or terminations from the program.
Q: Who can determine if an individual is a patient of a covered entity?
A: Section 602 of the Veterans Healthcare Act of 1992 provides guidelines for determining if an individual is a “patient” of a covered entity:
- The covered entity has established a relationship with the individual, such that the covered entity maintains records of the individual’s healthcare
- The individual receives healthcare services from a healthcare professional who is either employed by the covered entity or provides healthcare under contractual or other arrangements (e.g., referral for consultation) such that responsibility for the care provided remains with the covered entity
- The individual receives a health care service or range of services from the covered entity which is consistent with the service or range of services for which grant funding or Federally-qualified health center look-alike status has been provided to the entity. Disproportionate share hospitals (DSH) are exempt from this requirement.
Q: What are ICyte’s capabilities to monitor 340B violations?
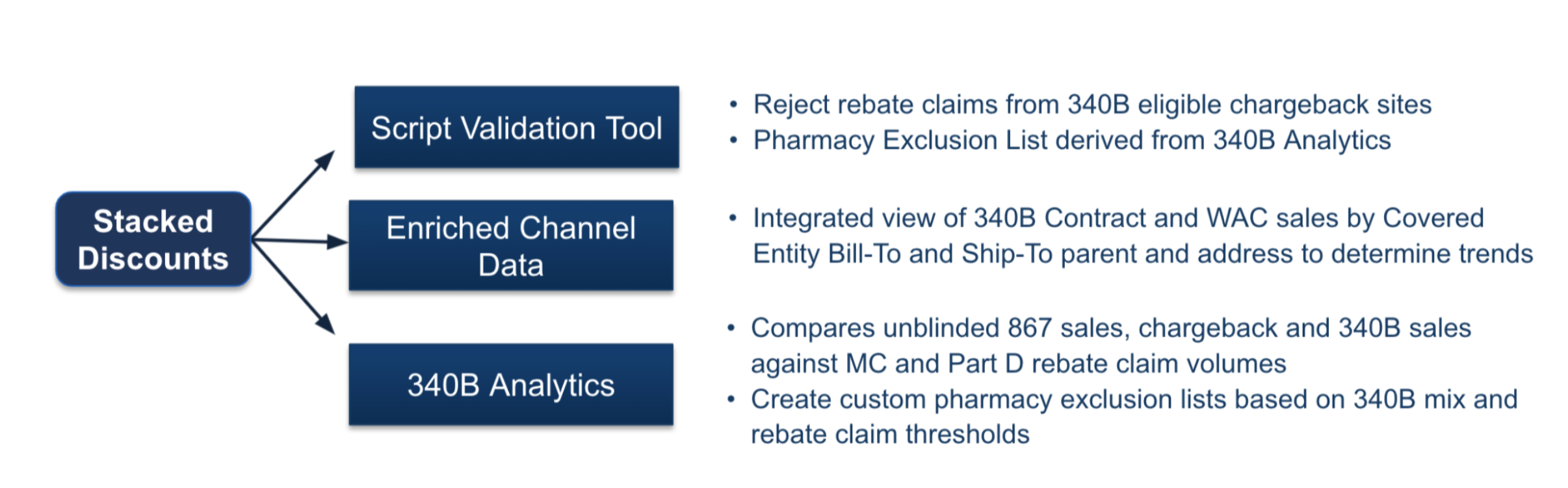
A: As a managed service and within our CLD system, we actually can provide within the claim level details the ability to validate for duplicate discounts. Another capability is within our chargeback system, where now you can reject chargebacks that are processed against a 340B covered entity or contract pharmacy if the manufacturer’s policy allows.
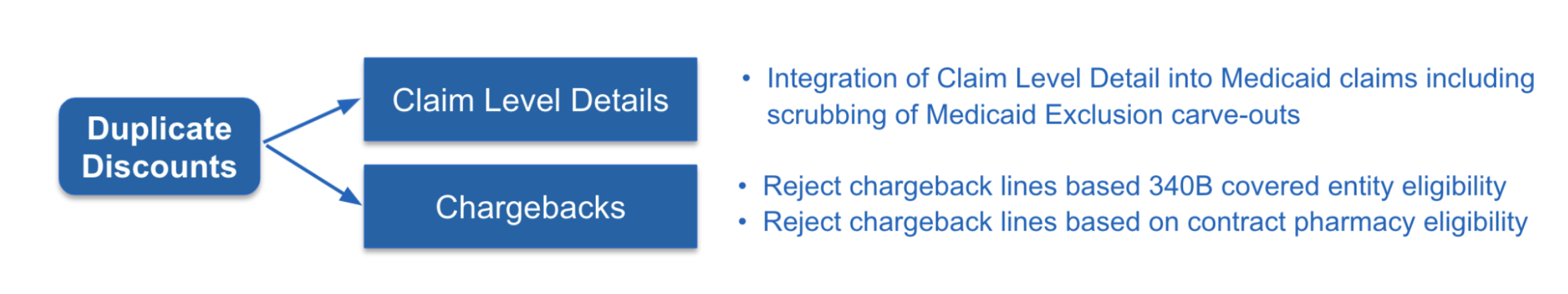
It’s not just CLD, we can do stack discounts in script validation tool. This is the holy grail for manufacturers: everything is in one unified product stack, where our teams could take all of the results of channel data and make a pharmacy exclusion list that’s passed into the script validation tool to reject all the claims based upon what we see in the channel data.